ПРОДУКТ
D-Xylose
| Chemical Name | : D-Xylose |
| Category | : |
| Specification | : USP |
| HS Code | : 2940.0000.00 |
- Телефон: +86-532-83876123
- Факс: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
ХИМИЧЕСКАЯ ХАРАКТЕРИСТИКА
| Structure Formula | : 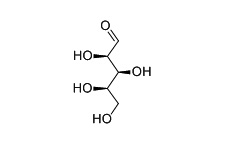 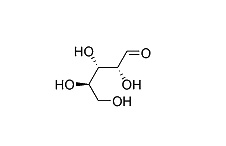 |
| CAS Number | : 6763-34-4; 31178-70-8 |
| Molecular Formula | : C5H10O5 |
| Usage | : No calorie sweetener. Used in obesity and diabetes. Also for fat oxidation prevent dose, preparing Caramel material and through Maillard reaction for preparation of pork and other spices. Xylose on human intestinal bifidobacteria have higher proliferative effect, consumption of xylose can improve the body's micro environment. Improve the body's immune ability. The compatibility of xylose and the food is very good, the addition of small amounts of xylose in food, can reflect the good health care effect. Xylose at the same time with calcium intake, can increase the body's calcium absorption rate and retention rate, but also to prevent constipation. For the preparation of xylitol. Because of its flavor effect is obvious, can be used in perfumes and pet feed industry Due to its efficient cause Maillard reaction, so it can be used in the production of food flavoring agent Because of its coloring effect is obvious, can be used in the food industry golden brown coloring, such as butter and bread coloring. Pharmaceutical raw materials and pharmaceutical intermediates Suitable for the preparation of flavors and fragrances |
ТЕХНИЧЕСКИЙ ПАРАМЕТР
| Item | Standard |
|---|---|
| Appearance | White crystals or white crystalline powder, no odor, freely soluble in water, no foreign matter. |
| Appearance of solution | Solution is clear and colourless |
| Purity | ≥98.5% |
| Transmittancy | ≥96% |
| Moisture | ≤0.30% |
| Loss on drying | ≤0.50% |
| Residue on ignition | ≤0.05% |
| Specific rotation | +18.5~+19.5° |
| PH | 5.0-7.0 |
| Acidity or Alkalinity | ≤0.2ml NaOH 0.1mol/l |
| Chloride | ≤0.005% |
| Sulfate | ≤0.005% |
| Sulfate Ash | ≤0.1% |
| Heavy metals(Pb) | ≤0.002% |
| Iron | ≤0.0005% |
| Total plate count | ≤3000 CFU /g |
| Coliform | ≤30 MPN/100g |
| Pathogenic Bacteria (Salmonella,Shigella, Staphylococcus Aureus) | Negative |
| CONCLUSION | WE CONFIRM THAT THE GOODS ARE QUALIFIED |
ЗАРЕГИСТРИРОВАННЫЕ ДОКУМЕНТЫ
The available documents are:
Chinese GMP
CEP
DMF/CTD format
The available registered countries are:
Algeria
Spain
Australia
Chinese GMP
CEP
DMF/CTD format
The available registered countries are:
Algeria
Spain
Australia